

An aging society demands new approaches to treatment across all fields of medicine.
For example, the growing population of osteoporosis sufferers is making early treatment of bone fractures a pressing issue. Another issue demanding attention is the shortage of potential donors for organ transplants to treat liver failure and other serious diseases. Research is progressing on how to regenerate organs using biological scaffolds as an alternative to transplantation.
Cancer is the leading cause of death among Japanese. Surgery and radiation therapy are widely used to treat this disease. However, the heavy physical burdens imposed on patients by these approaches are creating a need for less invasive techniques. Professor Aizawa comments: “We see new biomaterials playing a potentially major role in the establishment of novel high-tech approaches to treating cancer.”
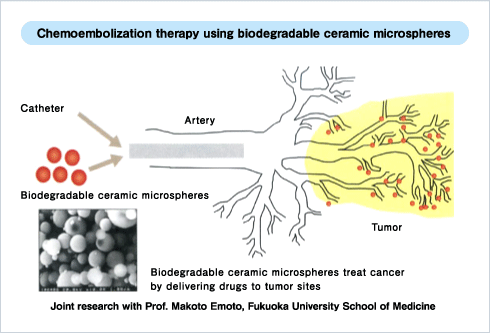
For instance, materials suitable for use in drug delivery systems are key development targets. The novel idea, combined chemical therapy with embolization, is to deliver bio-absorbable ceramic microspheres filled with drugs directly to the site of the tumor via a catheter.
Professor Aizawa currently leads a team of researchers who are focused on the development of high-performance biomaterials to support advanced medical technologies and related medical device applications.
In fiscal 2006 the Ministry of Education, Culture, Sports, Science and Technology selected Meiji University’s high-performance biomaterials development project as an Academic Frontier Project. Core project themes include early treatment of bone fractures, organ regeneration and advanced cancer treatments.
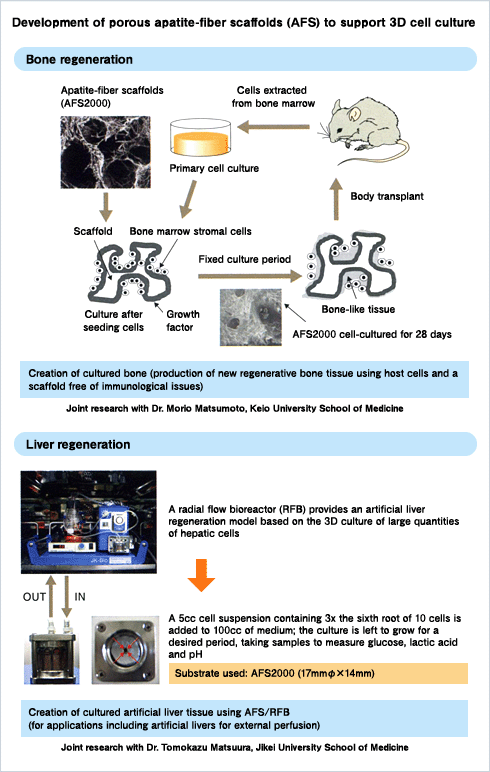
Use of Apatite in Bone and Organ Regeneration
In his research, Professor Aizawa studies apatite fiber, a naturally occurring biomaterial that belongs to the family of phosphate minerals.Apatite is known as one of the ingredients in toothpaste. It also has potential applications in the development of artificial bone and liver regeneration.
“As an alternative to transplantation, we are using apatite fibers as scaffold materials to create or regenerate the target tissue that makes up bone or a specific organ,” explains Professor Aizawa. “These scaffolds provide a place for the cells to grow and regenerate tissue. We encourage cell growth by adding growth factors and the resulting tissue can be used to create a bone or a liver.”
For example, making artificial bone involves bathing apatite-fiber scaffolds (AFS) in a suspension of mesenchymal stem cells to make the cells adhere to the AFS. A bone morphogenetic protein is then added as a growth factor to enable the creation of bone tissue.
“Think of the scaffold as a house and the cells as people,” says Professor Aizawa. “We add the growth factor to encourage the people to move into the house.”
Previous research into bone regeneration has focused on using collagen or biodegradable polymers as scaffolds. The problem with these materials is that they have tended to absorb too quickly or else resist the formation of hard tissue. Apatite fiber solves these problems.
The scaffolds must fulfill several conditions to support the regeneration process. First, they must provide an environment similar to that inside the body. Second, they must enable normal activation of cell functions. Third, they need to have mechanical strength. Lastly, they must be capable of replacing actual body tissue.
Apatite-fiber scaffold (AFS) is an extremely porous substance with a porosity of over 98%. Its structure allows cell cultures to develop in three dimensions. The result is that tissue formed on an AFS generally behaves in the same way as cellular tissue inside the body, which makes it ideal as a substrate for rapid regeneration or restoration of bone tissue. Apatite-fiber scaffold combines the potential for cell cultivation with mechanical strength, despite its high level of porosity.
“It is also soluble, which makes it an excellent multi-purpose material for use in making bone, as well as various organs,” says Professor Aizawa.
The research program has scored a number of successes to date. These include biomaterial scaffolds for use in regenerative medicine, organic-inorganic hybrid composite materials having a similar mechanical strength to natural bone, ceramic microspheres for use in targeted chemoembolization therapy, and bone-repair cement.
Preparing for the Super-aged Society
The professor is motivated to continue his research by the knowledge that success will produce technology capable of helping people.He explains: “I chose this field when a professor gave me an interesting research theme while I was studying at university. What fascinated me was that it was an interesting field that could also prove helpful for people. Today, I am collaborating with companies to develop materials that will enter commercial use. I want to continue making materials for the benefit of patients.”
There remains much to do in his chosen area, and the professor does not expect to lose interest in the field. He also hopes that his research students will be proactive and think for themselves.
“Our ceramic microspheres filled with drugs can shut off the blood flow to a tumor by narrowing the blood capillaries around it. This can shrink the tumor. We are also looking at a number of related bioengineering research projects with medical potential. I hope that students interested in these sorts of things will join our team,” he says.
Quality of life will be at the top of the agenda in a future super-aged society. Materials development promises to make a major contribution to the field of medicine. Such materials have the potential to make life more comfortable for many seniors.
“The technology that I have helped to develop may even benefit me when I am older,” says Professor Aizawa. “So I want to continue developing materials for the future that could help to ease the burden of medical treatments for many patients.”
Profile
Professor Mamoru Aizawa, Department of Applied Chemistry, School of Science and Technology, specializing in biomaterials, tissue engineering, ceramicsBorn in Yokohama City, Kanagawa Prefecture in 1968. Graduated from the Chemistry Department, Faculty of Science and Technology, Sophia University. Completed the pre-doctorate course in Applied Sciences, the Faculty of Science and Technology, Sophia University. Conferred with a doctorate degree (engineering) by Sophia University.
After working as a researcher in Material Research Laboratories, Kao Corporation, worked as a research fellow in the National Industrial Research Institute of Nagoya, Agency of Industrial Science and Technology, Ministry of International Trade and Industry, and the Cambridge Center for Medical Materials, Department of University of Cambridge, was a visiting researcher at the Department of Materials Science and Metallurgy, the University of Cambridge (under the Sophia University overseas research program), a research associate in the Department of Chemistry, Faculty of Science and Technology, Sophia University, and an associate professor in the Department of Applied Chemistry, School of Science and Technology, Meiji University. Has been in his current position since 2008.
Society memberships
Vice-Chairperson of the Division of Ceramics in Medicine, Biology and Biomimetics, The Ceramic Society of Japan, Director in Charge of Public Relations at the Japanese Association of Inorganic Phosphorus Chemistry, member of the Council of the Society of Inorganic Materials, Japan, Japanese Society for Biomaterials, and othersMajor publications
peer-reviewed academic papers (128 others not listed here)1) M. Honda, T. Fujimi, S. Izumi, K. Izawa, M. Aizawa, H. Morisue, T. Tsuchiya, N. Kanzawa, “Topographical analyses of proliferation and differentiation of osteoblasts in micro- and macro pores of apatite-fiber scaffold”, J. Biomed. Mater. Res.: Part A, 94A, 937-944 (2010).
2) M. Emoto, Y. Naganuma, B. Choijamts, T. Ohno, H. Yoshihisa, N. Kanomata, M. Aizawa, “Novel Chemoembolization Using Calcium-phosphate ceramic microsphere incorporating TNP-470, anti-angiogenic agent”, Cancer Sci., 101, 984-990 (2010).
3) T. Kawasaki, Y. Niki, T. Miyamoto, K. Horiuchi, M. Matsumoto, M. Aizawa, Yoshiaki Toyama, “Negative modulation of BMP-2-induced osteoblast differentiation by HGF is affected by the timing of HGF administration”, Biomaterials, 31, 1191-1198 (2010).
4) H. Morisue, M. Matsumoto, K. Chiba, H. Matsumoto, Y. Toyama, M. Aizawa, N. Kanzawa, T. J. Fujimi, H. Uchida, and I. Okada, “In vivo bone formation using three-dimensional scaffolds developed from a single crystal apatite fiber”, J. Biomed. Mater. Res. A, 90A, 811-818 (2009).
5) M. Aizawa, A. E. Porter, S. M. Best and W. Bonfield, “Ultrastructural Observation of Single-crystal Apatite Fibres”, Biomaterials, 26, 3427-3433 (2005).









